Chemical Name:Chloramphenicol
CAS No.: 56-75-7
Molecular formula: C11H12Cl2N2O5
Molecular weight: 323.13
Related categories:Antibiotics, alkaloids; Active Pharmaceutical Ingredients;
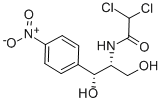
Chemical properties :
White or yellowish with green needle crystals. Melting point 150.5-151.5 ° C (149.7-150.7 ° C). It can be sublimated under high vacuum. Slightly soluble in water (2.5mg/ml, 25°C), slightly soluble in propylene glycol (150.8mg/ml), soluble in methanol, ethanol, butanol, ethyl acetate, acetone, insoluble in ether, benzene, petroleum ether, Vegetable oil. Very bitter taste.
Physical and chemical properties:
Chloramphenicol, also known as ketamine, is a broad-spectrum antibiotic produced by Streptococcus mutans that inhibits the growth of bacteria. Natural chloramphenicol is a left-handed body (also known as levomycin). The synthetic product is white or yellowish needle-like or flaky crystal, odorless, very bitter taste, slightly soluble in water, ether and chloroform, soluble in methanol, alcohol, acetone or ethyl acetate, insoluble in benzene and petroleum ether. It is relatively stable in neutral or weakly acidic aqueous solutions and is prone to failure in the presence of alkali. The synthetic product is a racemate, also known as doxorubicin. Mycomycin is a mixture of chloramphenicol left-handed and right-handed bodies. Because the right-handed body has no antibacterial effect, the therapeutic effect of the synthetic drug is only half of that of the natural product. Chloramphenicol has inhibitory effects on Gram-negative bacteria and Gram-positive bacteria, and can be used for the treatment of typhoid fever, urinary tract infection, whooping cough, pneumonia, sepsis and the like. However, it is harmful to the liver, and the method and dosage should be strictly in accordance with the doctor's advice. Chloramphenicol palmitate is insoluble in water, oral and tasteless, also known as odorless chloramphenicol. After being administered, it is decomposed by fat and hydrolyzed by the small intestine to release chloramphenicol. The odorless chloramphenicol contains about 60% chloramphenicol, so the dose should be increased by 2/3 when taken. Especially suitable for children. An ointment containing 1% chloramphenicol can be used for the treatment of infections of the eyes and skin. In agriculture, it is mainly effective for plant bacterial diseases, such as rice bacterial blight, which has strong systemic action and is safe for plants.
Uses:
It is a broad-spectrum antibacterial antibiotic. It is the first choice for the treatment of typhoid fever and paratyphoid fever. It is one of the special drugs for the treatment of anaerobic infections, and secondly for the treatment of various infectious diseases caused by sensitive microorganisms. As adverse reactions are severely used less and less.
production method :
A large number of studies have been carried out on the production methods of chloramphenicol in various countries in the world, which are summarized as follows: (1) p-nitroacetophenone method; (2) styrene method; (3) cinnamyl alcohol method; (4) p-nitro group Cinnamyl alcohol method; (5) p-nitrobenzaldehyde method. China adopts p-nitroacetophenone method, which is obtained by nitration, oxidation, bromination, salt formation, hydrolysis, acetylation, addition, reduction, decomposition, separation and dichloroacetylation of ethylbenzene.