Chemical Name:Sodium hydroxide
CAS No: 1310-73-2
Molecular formula: HNaO
Molecular weight: 39.99711
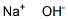
Nature
Melting point | 681 °C(lit.) |
Boiling point | 145 °C |
Density | 1.515 g/mL at 20 °C |
Vapor density | <1 (vs air) |
Vapor Pressure | 1 mm Hg ( 745 °C) |
Refractive index | 1,473-1,475 |
Flash point | 176-178°C |
Storage conditions | 2-8°C |
Solubility | H2O: 1 M at 20 °C, clear, colorless |
form | beads |
colour | White |
PH value | >14 (100g/l, H2O, 20℃) |
Water solubility | SOLUBLE |
Sensitivity | Air Sensitive & Hygroscopic |
Decomposition | 176-178 oC |
Merck | 14,8627 |
stability | hygroscopic |
CAS database | 1310-73-2(CAS DataBase Reference) |
Chemical properties
Pure products are colorless and transparent crystals. Soluble in water, while strongly exothermic. It is soluble in ethanol and glycerin; it is insoluble in acetone and ether. The dew is placed in the air and will eventually dissolve completely into a solution.
Use
1. Basic chemical raw materials, used as high-purity reagents, widely used in chemical, metallurgy, paper, petroleum, textile and daily chemical industries.
2. Used as an analytical reagent, a saponifier, a small amount of carbon dioxide and water absorbent, and also used in the manufacture of sodium salts.
3. Used in textile, printing and dyeing, enamel, paper, synthetic detergent, pesticide, metallurgy, food, rubber and other industries
4. Basic chemical raw materials, widely used in chemical, metallurgy, paper, petroleum, textile and daily chemical industries
5. Calibration for acid use in analysis
6. Mainly used in mucilage rayon, synthetic fiber, dye intermediate, rubber regeneration, fabric bleaching, paper making, soap making, leather making, etc.
7. As a neutralizing agent for acid, it can be used for peeling fruit processing, or as a detergent for empty bottles, empty cans, etc., and used in an appropriate amount according to production needs.
8. Basic analytical reagents for a wide range of applications. Prepare standard lye for analysis. A small amount of carbon dioxide and moisture absorbent. Neutralization of acid. Sodium salt manufacturing.